The Unsung Heroes Who Ended a Deadly Plague
Chickenpox (Varicella) Vaccine For Adults
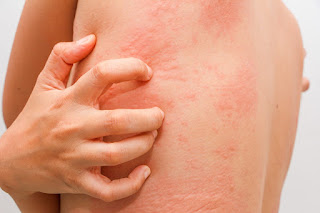
Chickenpox is a common illness caused by the varicella-zoster virus. Symptoms of chickenpox include fever and itchy spots or blisters all over the body. Chickenpox is usually mild and runs its course in five to 10 days, but it can cause more serious problems when teens and adults get it. People with weakened immune systems are especially susceptible to developing serious complications from chickenpox.
Some complications that can arise from chickenpox include:
Vaccination is the best way to prevent chickenpox. A chickenpox vaccine has been available in the U.S. Since 1995 and is easy to get from a doctor or a public health clinic. The chickenpox vaccine is very effective at preventing the disease -- between 70% and 90% of people who get vaccinated will be completely immune to chickenpox. If a vaccinated person does get chickenpox, the symptoms will be very mild and only last for a few days.
All adults who have never had chickenpox or received the vaccination should be vaccinated against it. Two doses of the vaccine should be given at least four weeks apart.
If you've never had chickenpox or been vaccinated and you are exposed to chickenpox, being vaccinated right away will greatly reduce your risk of getting sick. Studies have shown that vaccination within three days of exposure is 90% effective at preventing illness; vaccination within five days of exposure is 70% effective. If you do get sick, the symptoms will be milder and shorter in duration.
You should not be vaccinated against chickenpox if you:
These people should check with their doctor about getting the chickenpox vaccine:
The chickenpox vaccine is made from a live, weakened form of the varicella virus. That means the virus is able to produce immunity in the body without causing illness.
The most common side effect from the chickenpox vaccine is swelling, soreness, or redness at the site of the injection. A small number of people may also develop a mild rash or a low-grade fever after vaccination.
Serious reactions to the chickenpox vaccine are extremely rare, but they may include:
If you think you may be having a serious reaction to the chickenpox vaccine, call your health care provider right away. Make a note of the symptoms you're experiencing, and report them to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967.
Women who receive the chickenpox vaccine during pregnancy should contact their health care provider right away. Chickenpox during pregnancy can cause birth defects, so there may be a risk that the chickenpox vaccine could cause the same birth defects.
As with other vaccines, the risks associated with the chickenpox vaccine are much lower than the risks associated with the disease itself.
Proof That Nixing Vax Dissent Backfired
As a primary care physician, I administer vaccines on a regular basis, from flu to tetanus to pneumonia to shingles.
I bring these vaccines out during doctor visits, and I discuss the pros and cons with my patients while the vaccines are being displayed.
I am completely open about potential side effects.
The core vaccine conversation between doctor and patient is a risk/benefit analysis.
It's a discussion, one in which the patient decides.
One of the big mistakes made by the Biden administration with the COVID vaccines is not having this discussion.
Rather than keeping expectations realistic — telling folks that the vaccine would lessen the severity of COVID, instead of preventing a patient from getting it — the administration forced as many as it could to get the jab through mandates.
Instead of being up front that there was a slight risk of side effects, the administration put pressure on social media companies to censor any discussion.
Now we have research data from the Global Vaccine Data Network that detail exactly what risks accompany the COVID vaccines.
Start your day with all you need to knowMorning Report delivers the latest news, videos, photos and more.
Thanks for signing up!
The study found what has been shown previously in smaller studies — that side effects from the vaccines are mostly mild, but they aren't non-existent.
The study showed an association between the mRNA vaccines and rare myocarditis, and between the Oxford Astra Zeneca vaccine — which was never approved for use in the United States — and pericarditis, Guillain-Barre syndrome and other neurological complications.
The Moderna mRNA vaccine was also associated with a slight increased risk of brain swelling.
Conspiracy theorists will take this as vindication.
It isn't, for the simple reason that the study also determined that the risk of a neurological problem from COVID was more than 600 times higher than from the vaccine.
But such was the mistake of the Biden administration's squashing of dissent that any discussion of side effects makes them look like liars.
The truth is that COVID mRNA vaccines have side effects, including rarely myocarditis, but studies have also shown that they decrease the risk of myocarditis from COVID itself.
The benefits of the vaccines far outweigh the risks, particularly for older Americans.
It's a discussion — not a demand — we should have had from the beginning.
A discussion that would have been easier had the White House asked primary-care doctors to be the ones to discuss and administer the vaccine.
The vaccine mandates only helped to sow distrust in government, and lowered vaccine acceptance in general.
Which is devastating because vaccines for children, particularly for measles and mumps, have saved millions of lives.
Plus, the public drive to squash any criticism of the vaccine along with downplaying side effects helped lead to a blossoming of conspiracy theories, anger and confusion.
This should be a real lesson for any administration going forward.
Marc Siegel, MD, is a clinical professor of medicine and medical director of Doctor Radio at NYU Langone Health and a Fox News medical analyst.
Switching Arms For Vaccines Could Help Boost Your Immunity, Study Finds
The COVID-19 vaccine is still relatively new, and researchers are still interested in studying how to maximize its effectiveness.
Typically, people receive the COVID-19 vaccine in the upper arm, which has multidose options. Multidose vaccines can be received in the same or different injection site for each dose. Other examples of multidose vaccines include those for measles mumps and rubella (MMR) and shingles.
A recent study published in The Journal of Clinical Investigation examined whether switching arms for two doses of the Pfizer BioNTech COVID-19 vaccine increased effectiveness.
Participants who switched arms for vaccine doses experienced a higher antibody response than those who received doses in the same arm.
The results showed this response increased over time in the subsequent follow-up visits.
These results point to a simple way to increase vaccine effectiveness. Future research could explore whether switching injection sites for other multidose vaccines could help improve immunity.
COVID-19 vaccination has effectively slowed infection rates and helped reduced severe illness.
The two main COVID-19 vaccines are both mRNA vaccines produced by Pfizer and Moderna.
Current recommendations from the Centers for Disease Control and Prevention (CDC) involve single doses of the Moderna or Pfizer-BioNTech vaccines for people who are not immunocompromised. However, multiple doses are still recommended for people who are immunocompromised.
Previously, other individuals received two doses of the Pfizer-BioNTech vaccine. Researchers of the current study wanted to see if the immune response produced by the Pfizer-BioNTech vaccine differed based on whether or not participants received doses in the same arm or the opposite arm from their initial dose. Study authors note there hasn't been a lot of research conducted in this area.
Researchers included participants from the OHSU COVID-19 Serology study, including almost 950 adults in their analysis. A total of 507 participants received at least two doses in the same arm, and 440 received at least two doses in opposite arms.
Researchers also looked at antibody response in a subgroup of matched pairs, with each pair having similar age, gender, vaccination, and time intervals between blood sample testing.
They were able to follow up on immune response among participants for up to 14 months after boosting.
Overall, researchers found that the group receiving vaccination doses one and two in opposite arms had a better immune response than those receiving doses in the same arm.
They saw higher levels of SARS-CoV-2 specific serum antibodies. They observed this difference more with later immunity testing than with earlier testing.
Study author Dr. Marcel E. Curlin, associate professor of medicine in the division of infectious diseases at the Oregon Health and Sciences University and the medical director for occupational health at OHSU, noted the following to Medical News Today:
"In the context of first-time receipt of a 2-dose vaccine regimen, antigen–specific antibody levels resulting from vaccination are higher when giving the second dose in the contralateral arm relative to the first dose. This effect is durable, lasting more than a year after boosting. Contralateral vaccination also results in a "broader" immune response to challenges slightly different from the original vaccine (for example, to a variant of the original virus). We do not yet understand why this happens, but it is likely related to formation of memory and multiple rather than individual lymphoid centers."
Non-study author Dr. Arturo Casadevall, PhD, a microbiology and immunology expert with Johns Hopkins Medicine, told MNT the study data are strong.
"The finding that contralateral arm vaccination results in higher antibody responses suggests that the simple intervention of switching arms during initial vaccination and boosting could produce stronger immunity and perhaps longer lasting protection," he shared.
"This is an example of simple medical research with potentially high benefits for the individual and for public health."
Despite the promising implications the new research does have certain limitations.
First, researchers acknowledge the potential bias that could have occurred, though they believe this cannot account for all the results seen. Second, this study looked at a specific type of vaccination among adults and did not examine alternative immunization routes, so the results may not apply or be significant for other areas.
Researchers also did not look at cellular immunity when looking at potential protection from severe illness.
In addition, the cohort was comprised of healthcare workers, a specific population, so more research could also include more individuals in other fields.
Only 23% of participants were male, so it's also possible for future research to include more gender balance. There was also a limitation based on how many participants completed all follow-up appointments.
Non-study author Jessica Smith Schwind, PhD, MPH, director at the Institute for Health Logistics & Analytics and associate professor of Epidemiology, Georgia Southern University, said from an epidemiologic perspective, the study has the potential to influence standard practice, but shared a word of caution:
"However, a randomized study will be the gold standard to determine if a contralateral administration of the vaccine series would be most beneficial (and to what extent) for mRNA COVID-19 vaccinations. Also, it is important to keep in mind that immunologic response is a multi-faceted, complex process that can be measured in different ways. This study only measured antibody titers, which is only one component that influences a person's overall immune response to a pathogen."
Future research could focus on verifying these initial findings and expanding the data collection, such as looking at additional time points after vaccination.
At a basic science level the observation raises new questions for immunological research since it is difficult to explain how this effect occurs based on current understanding of how immune responses develop," Dr. Casadevall said.
"I think the next step would be to carry out a prospective randomized controlled trial to determine if the effect holds. If the findings are replicated, I can imagine that this could lead to changes in clinical practice for how vaccines are administered and would stimulate new basic science research to understand the immunological mechanisms involved," Dr. Casadevall noted.
This research opens the door for future research into maximizing vaccine effectiveness.
"We can probably derive greater levels of protection elicited by vaccines, based on the way we provide vaccination," Prof. Curlin noted.
"Improved protection would likely be in the form of some degree of decrease in disease severity, particularly those with comorbid illness who are likely susceptible to severe disease."
One area for future research is looking into how switching vaccination sites may apply to other multidose vaccines. For example, the boost in immunity seen in this study may hold true for other multidose vaccines and increase their overall effectiveness.
Researchers note that future research can include pediatric data, as many of these multidose vaccines are usually part of child vaccination regimens.
Professor Curlin noted the potential benefits of this line of research in the future:
"This effect, if generalized, could change the way we administer certain vaccine regimens, particularly in children. This effect could [also] have an impact on vaccines in development, particularly those with efficacy near threshold cutoffs for viable vaccine products. [However], it is important to remember that this issue requires additional study to help us better understand the mechanism for this effect and its generalizability to other vaccines."


Comments
Post a Comment